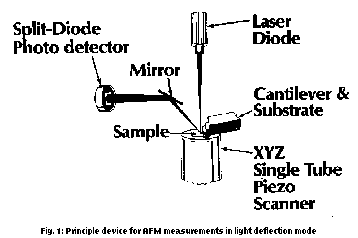
Atomic Force Microscopy
AFM for the elucidation of solid state syntheses which avoid wastes
Prof. Dr. Gerd Kaupp, Dipl.-Chem. Jens Schmeyers,
Dipl.-Chem. Michael Haak, Cand.-Chem. Andreas Herrmann
Labo 1995 6 57
Article installed with permissions of the publisher
WWW-adaption by M. Haak
The AFM-technique provides new answers to the question of organic solid state reactivity and it uncovers unexpected mechanistic details, which cannot be observed by other analytical procedures. The new concept of phase rebuilding provides comprehension of the longrange anisotropic molecular movements on the basis of crystal packing data. Previously such movements thought impossible and were not forseen, but they now prove essential for the chemical reactivity. Thus a theoretical comprehension of surprisingly efficiant waste-free techniques of syntheses is provided for the first time and such syntheses have been developped up to the kg scale already. Examples for gas/solid- and solid/solid-reactions have been chosen from various branches of organic chemistry. All of them run uniformly and quantitatively and are clearly superior to the corresponding liquid phase reactions with their waste problems. Therfore gas/solid reactions are not only able to detoxicate atmospheric air or exhaust gases but are particularly important in view of their potential as primary action for the avoidance of wastes in industrial chemical production. Both gas/solid- und solid/solid-reactions are particularly simple and cost-effective because they avoid solvents and liquid phases. AFM helps in finding systematizing and predicting further examples by indicating the reasons for the success of these synthese techniques for the future.
Imaging of individual atoms is epoch-making. For applied AFM it is important to scan in the nm- and µm-range if the surface structures happen to occupy that range. Both physical properties and chemical reactions may depend on submicroscopic surface structures. The requirements for the scanning of large, steep, densely packed, deeply penetrating nanostructures with AFM have been summarized [4]. In Ref.[4] is also shown how changes of the surface during the scanning are avoided, how artefacts are recognized, and how the images look like if nanoliquids form at the surface or if the limits inherent to the AFM techniques are surpassed, an example being the imaging of membrane pores. Waste-free solid state syntheses which do not use solvents are the future production techniques, undoubtedly. Solid-state photolyses are longer used already [3] however, their large scale technical use poses practical problems.
Much more interesting are gas/solid- [5] and solid/solid-syntheses [6]. Both of these deserve puplic support and publicity. The development of such techniques was hampered, because incomplete theories of the so called topochemistry [7] were not able to "understand" the occurrance of such reactions. Despite the fact that most reactions of the new techniques for syntheses proceed quantitatively and avoid all kinds of waste (several examples have been performed in the kg scale) the opinion still prevails, that one should rather dissolve the reactants even at the expense of usually lowered yield and unnecessary problems with waste. Therefor it is very favorable that the investigation of organic gas/solid- [5] and organic solid/solid-reaktions [8] with the AFM uncovered unexpected new theoretical foundations. Thus primary actions may now be accepted which avoid waste problems because there is not only an experimental basis but also a theoretical one.
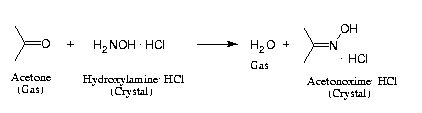
Reaction Scheme 1

Fig. 2 excellent quality (GIF 244 K) or Fig. 2 (JPEG 55 K)
Figure 2 shows both the hydration and the reaction of the surface hydrate with acetone vapor at short exposure. It can be seen, that the hydration forms very steep surface structures with heights of more than 1 µm. These features do not exhibit any asymmetries and are thus free of artefacts, at least in their upper parts [4]. However,the structures stand so densely (about one per µm2), that the rather large standard tip cannot reach the ground without its pyramidal faces being touched by the features. That circumstance is not seen in Figure 2b (and even less so in 2D-projections of it), but very clearly in the inverted perspective image (Figure 2d) which is most easily obtainable. In the inverted image the tops are pointing down and the depths are pointing up. The sharp edges which appear show that the pyramidal faces and the edges of the tip had contact with the cones (or even columns ?) on the surface while it tried to descend into the depths. Sometimes, if there was simultaneous contact from all sides a true image of the pyramidal tip is seen in Figure 2d (therein enlargement of the Z-scale!). It is to be concluded, that the surface features in Figure 2b are both taller and higher than can be traced with a standard AFM-tip. The advantages of perspective imaging over 2D-projections (which cannot image such tip/surface convolutes) are evident. The reactions of the (hydrated) hydroxylaminehydrochloride surface with acetone vapor leads to massive structures which are more than 4 µm high and exhibit the heights and valleys type (Figure 2c). The molecular movements that have occurred are very far-reaching when compared with molecular dimensions. The gas/solid reaction is unusually effective and the stability of the AFM-images during more than 10 consecutive scans proves that no liquid or nanoliquid phases occur [4].Figure 2: AFM surface of hydroxylaminehydrochloride; a: main surface after 20 min manipulation in moist air; b: after 200 min hydration in moist air; d: same as b but inverted image; c: after 2 min application of diluted acetone vapor on the surface hydrate of hydroxylaminehydrochloride.
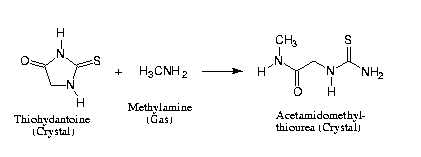
Reaction Scheme 2
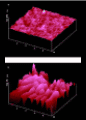
Figure 3: Natural (110)-AFM-surface of thiohydantoine; a: fresh; b: after repeated treatment with gaseous methylamine
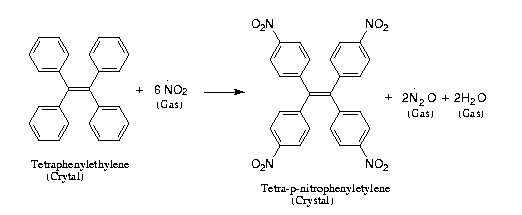
Reaction Scheme 3

Figure 4: AFM-surface of tetraphenylethylene; a: fresh natural (10-1)-surface; b/d: after 10 min exposure to 0.2 bar of NO2; c: after 20 min exposure to 0.2 bar of NO2;
Upon longer exponere to NO2 gas the circumwalled craters disappear, no doubt, as a consequence of phase
transformation into the product lattice. The initially formed cyclic
structures are again comprehended by correlation with the crystal structure
of the starting material [11]. The tetraphenylethylene molecules which stand
vertical under (10-1) have a limited possibility for partial turns around
their long axis. They use it if they grow thicker upon nitration. By doing
that they collide with next row molecules which then turn in opposite
direction and provoke a third and then a forth molecule to the corresponding
turns with finally filling the hole left from the first of these molecules.
Thus, a concentric process leaving a hole at its center is described. After
such initiation the growing molecules (consecutive nitrations) move upward
and present their bottom p-positions for nitration with NO2 gas. The crater
diameter is enhanced by participation of neighbouring rows of molecules and
those processes proceed until the original lattice breaks down [11]. Again,
the crystal structure dictates the long range anisotropic molecular movements
during phase rebuilding.
Contrary to solution diazotations, gas/solid diazotation may proceed
waste-free and allow elegant applications in syntheses [12]. The solid
state diazotation of sulfanilic acid to give its diazonium salt, which is
needed for the synthesis of widely used pH-indicator methylorange (via azo
coupling with N,N-dimethylaniline) provides particularly impressive
AFM-images.
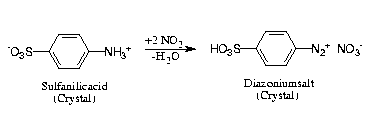
Reaction Scheme 4

Fig. 5 excellent quality (GIF 256 K) or Fig. 5 (JPEG 49 K)
Figure 5. (010)-AFM surface of sulfanilic acid monohydrate; a: fresh and barely effloresced; b/d: island formation after short diazotation with NO2-gas; c: after advanced diazotation by several treatments with NO2.
The initial surface in Figure 5a shows traces of efflorescence which may be the reason for the volcano like features. These are swallowed after short application of gaseous NO2 to the surface in air. Flat islands which may enclose little lakes are formed (Figure 5b, d). The characteristic island features are still recognizable after multiple application of NO2 (Figure 5c). However, the advanced height-growth is less uniform. A more roughened height scenery with embraced depths is optained. The formation of islands [10] is easily derived from the crystal structure of sulfanilic acid hydrate [13], which is depicted in Figure 6.
 Figure 6 (GIF 22K)
Figure 6 (GIF 22K)
Figure 6. Space filling stereoscopic representation of the molecular packing of sulfanilic acid monohydrate; top: view on the (010)-face showing hydrogen bridges between NH3+ and SO3- groups with additional H-bridging by H2O molecules; bottom: view on (100) which is orthogonal to (010) showing how the molecules alternate in their orientation and how they stand long-elongated under the (010) face which is on top; N: grid; S: shaded; O at S: circles; O of the water: dense circles.
The molecules stand vertically under (010) and their orientation alternates. Thus, a maximum of hydrogen bridges is obtained and the water molecules add further H-bridging. While the upmost layer on (010) is open for attack of the NH2 groups by NO2 of half of the molecules (Figure 6, top), the proceeding of the reaction into the crystal requires, that long elongated molecules exit from their H-bonded lattice positions and move upward in order to make the amino groups available for diazotation which pointed downward from the first layer and upward from the second layer etc. Clearly the reaction is hindered by this particular packing. Therefore the reaction spreads around the initial successfull invasions into row 2 etc. and thus from flat islands. 2 planes with a distance of 15 nm are present in Figure 5b/d.
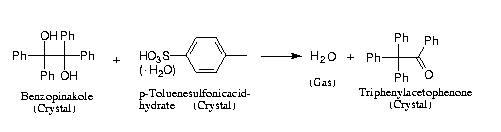
Reaction Scheme 5
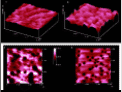
Fig. 7 excellent quality (GIF 292 K) or Fig. 7 (JPEG 57 K)
Figure 7: AFM-surface of benzopinacol (100); a: fresh in a distance of 0.5 mm from an onlaying crystal of p-toluenesulfonic acid monohydrate b/d: after 12 h at 50 °C, at a distance of 0.5 mm; c: after 12 h at 50 °C, at a distance of 0.1 mm;
The crystals are heated to 50°C for 12 h to initiate the reaction. They are remounted to the AFM and then scanned. One obtaines well-formed craters in 0.5 mm distance from the proton source (Figure 7b,d). Those are 5 mm deep and 150 mm wide. Importantly the reaction has much more proceeded it 0.1 mm distance (Figure 7c). The craters found there are about 11 nm deep and are mostly circumwalled with heights of typically 4 nm. Only at distances of more than 2 mm one finds surface images similar to the one in Figure 7a. The distance dependent reaction and crater formation can be understand again in terms of crystal data [8]. Here catalytic quantities of protons move over 2 mm from the small acid crystal and produce a charge separation, causing the crystals to stick together firmly. Mechanistically the protons combine with OH groups at the front side of molecular layers and water is liberated. Therefor, new protons are formed at the backside of cations after their rearrangement to yield triphenylacetophenone. These newly formed protons react with the frontside OH groups of the next layer etc. [8]. Interestingly, the morphologically dominant (001)-face does not react correspondingly, according to the AFM investigation. That surface remains unchanged after the same treatment and the acid crystal does not stick to that surface of benzopinacol. The reason for that is to be found in the orientation of the OH groups in the upmost layer of benzopinacol. On the (001)-face the hydroxyl-H (but not the free electron pairs) point outward. Therefor, no protonation can occur, inasmuch as there is also steric hindrance [8]. Conversely, on the reactive (100)-face, the free electron pairs are pointing outward and may thus be protonated [8]. This first mechanistic investigation of a crystal/crystal-reaction shows the potential of AFM and indicates a mechanistic understanding of the many further waste-free solid/solid-reactions which occur in most diverse types [6].
[1] G. Binning, C. Quate, C. Gerber: Atomic force microscope, Phys. Rev. Lett. 56 (1986) 930. [2] J. Frommer: Scanning Tunneling and Force Microscopy in Organic Chemistry, Angew. Chem. Int. Ed. Engl. 104 (1992) 1324. [3] G. Kaupp: AFM and STM in photochemistry including photon tunneling, Advances in Photochemistry 19 (1995) 119. [4] G. Kaupp, J. Schmeyers, U. Pogodda, M. Haak, T. Marquardt, M. Plagmann: AFM for the imaging of large and steep submicroscopic features, artifacts and scraping with asymmetric cantilever tips, Thin Solid Films, SXM1 special issue, 1995, in press. [5] G. Kaupp: Atomic force microscopy in organic gas/solid-reactions: How do the new phases build up?, Mol. Cryst. Liq. Cryst. 211 (1992) 1, cit. lit. [6] F. Toda in Y Ohashi (Hrsg.): Reactivity in Molecular Crystals, Kap. 4: Solid-to-solid organic reactions, Kodansha/VCH, Tokyo/Weinheim, 1993, S. 177. [7] M. D. Cohen, G. M. J. Schmidt, F. I. Sonntag: J. Chem. Soc. (1964) 2000; G. M. J. Schmidt: ibid. (1964) 2014; M. D. Cohen: Angew. Chem. 87 (1975) 439; Mol. Cryst. Liq. Cryst. 50 (1979) 1. [8] G. Kaupp, M. Haak, F. Toda: Atomic force microscopy and solid state rearrangement of benzopinacol, J. Phys. Org. Chem. S 95 H 12, in press. [9] G. Kaupp, U. Pogodda, J. Schmeyers: Gas/solid-reactions with acetone, Chem. Ber. 127 (1994) 2249; cit. lit. [10] G. Kaupp, J. Schmeyers: Gas/Solid-Reactions of Aliphatic Amines with Thiohydantoines: Atomic Force Microscopy and New Mechanisms, Angew. Chem. Int. Ed. Engl. 105 (1993) 1656. [11] G. Kaupp, J. Schmeyers: Gas/solid-reactions with nitrogen dioxide, J. Org. Chem. 60 (1995) Heft 16, in press. [12] A. Herrmann: Diploma Thesis, University of Oldenburg, 1995. [13] A. I. M. Rae, E. N. Maslen: The crystal structure of sulfanilic acid mono-hydrate, Acta Cryst. 15 (1962) 1285.
G. Kaupp: Fachzeitschrift für das Laboratorium GIT 37 (1993) 284-294 und 581-586.
 To the Homepage of AK Kaupp (Organische Chemie I)
To the Homepage of AK Kaupp (Organische Chemie I)
haak@kaupp.chemie.uni-oldenburg.de