In part 1 of this work it has been shown how the completely new technique AFM works and how its application to the photodimerizations with crystals of cinnamic acid leads to fundamentally new insights into the reaction mechanisms [1]. Perspective images which are unexpectedly resolved and rich in details of chemically reacting surfaces show that unforeseen transport phenomena are obtained which reach considerably further than the crystal lattice parameters. These transports are oriented so definitely and distinctly that hitherto unimaginable molecular interpretations are possible with the aid of crystal structures and quantum mechanical calculations. The perspective images which are obtained with NanoScope III Software (mix of colour height coding and illumination) and advanced video technique in phantastic quality (mixed colours from yellow, cyan, magenta) can be reproduced in four-colour lithography (yellow, green, blue, red colour spots) satisfactorily even though there are losses in purity and brilliance of the colours and the details of the originals (paper prints or large dias) become somewhat shallow. However, these losses are less severe than in the conversion to black and white images in which some of the important details will be scarcely recognizable any more.
In this part 2 we deal with the application of AFM to photochemical [4-4]-dimerization of anthracenes 1 which yield the compounds 2. The experimental details of the measurements with the NanoScope II are described in part 1 [1].
2.1. Photodimerization of Anthracenes
In the long known photodimerization of anthracenes in liquid solution there are obtained head-to-tail 9,10'/10,9'-products in most cases. However, there have also been instances where head-to-head dimers had been found, which were overseen previously. The solid phase photolyses of anthracenes charged the topochemical postulate for decades with hitherto unsolvable difficulties. All examples that contradicted the theory were without hesitation, though not very convincingly eliminated from the scope of topochemistry and termed as "crystal defect reactions". The "topochemically allowed" ones, however, were without further proof taken as support for topochemistry with the later provision that the dimerizations occur within "reaction cavities" in the bulk of the crystal. A summary of the argumentations is given in Ref. [3]. From the examples which are given in Formula 1 only 1b and perhaps 1c [4] formally meet with the requirements of topochemistry. The dimerization of anthracene 1a itself is "topochemically forbidden" (large distances) and even worse, 1d should yield the head-to-head dimer (not shown) in a "topochemically allowed" reaction according to crystal structure. It does, however, form the " wrong" head-to-tail dimer 2d instead (survey on the literature in [3]).

The AFM-images with anthracene 1a show clearly, that the photoreaction occurs in the surface regions and that there are long-range molecular regroupings.
The various terrace steps on the (001)-surface in Figure 1a are 1,2,3, and 4 nm high. This corresponds exactly to 1,2,3, and 4 molecular layers and constitutes a phantastic result. Despite their unimaginable minuteness, the chainy hills in Figure 1b grow exclusively along these molecular steps to considerable height. This continues upon further irradiation up to a maximum height of 50 nm (Fig. 1c), and finally one observes a thermal phase transformation: low floes with pronounced orientational preference are obtained as depicted in Figure 1d. The chemically changed layer is now easily detached mechanically. Such details could not be received at all with electron microscopic recordings or other techniques. The AFM-information is gratifyingly direct and immediately comprehensible.


According to crystal-lattice analysis (Fig. 2) the anthracene molecules 1a are interlocked (but not parallel or with short distances) in horizontal layers of 10 Å thickness and cut the (001)-face (P21/a) at a steep angle of 67°. Also in the "topochemically allowed" 9-methylanthracene 1b the molecules stand steeply (78°) on the dominant face ((100),P21/c). As a matter of fact, in both cases the dimer phases are formed as floes [3].
Clearly, as the molecules 1a at the molecular steps may be easily pushed aside, this possibility is used for release of internally generated pressure even though we literally deal here with only 1,2,3, or 4 molecular layers. That means, disturbance of the crystal lattice by dimer formation within these layers can be met with lateral transport, similar disturbance in the neighbouring layers by vertical and then lateral transport. This is the obvious basis for a molecular understanding, which, of course, will have to be refined by submicro X-ray diffraction at the hilly chains of 16 nm height (Fig. 1b). However, such facilities are not yet available. At any rate, far-reaching material transports are so important, that pretendedly too large distances in the range below 10 Å or so should no longer be disturbing. It is the phase rebuilding which clearly governs the process.
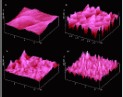
Our single crystals of 9-chloroanthracene 1c which were obtained as described could not be unambiguously assigned to one of its known modifications. However, the very different results on the morphologically dominant and on the side-face along the long edge are of highest importance. From the rather flat real main surface (crystals from hexane) very regular floes form. These grow up to 50 nm height and display an enormous sharpness (Fig. 3b). After 15 min irradiation the floes grow together and thus become thicker.
From the rather rough side-face beautiful volcanoes form all over (Fig. 3d). These are retained and twice as high after 15 min irradiation. Thus, there are no secondary phase transformations discernable in those cases. Further studies will have to await the X-ray structure analysis of 1c, unfortunately. However, it is remarkable already, that the phase rebuilding mechanisms and the nanostructure formations on the different crystallographic faces are particularly beautiful and different. Much will have to be learnt from that on the molecular transports over large distances, and more so, if additional crystal faces may be probed as well.


Interestingly, according to the AFM-measurements 9-cyanoanthracene 1d does not make it its own way, even though there is formation of the product 2d which does not correspond to the crystal structure. The succession of pictures in Figure 4 shows the development of floes strictly along the steps of the smooth natural surface by continuous irradiation. Thus, the new features do not grow on (010) but on the faces which stand on that surface. From there the phase rebuilding works itself ahead on the (010)-basis and well-organized floes are obtained. Such behaviour could not be thought of before. What is seen in Figure 4, is direct experimental evidence. Thus, there is no exceptional mechanism in this reaction. Nevertheless, in the formation of 1d either one of the reaction partners would have to turn around by 180° or it should visit the neighbouring molecular layer. This is shown by the crystal packing diagram in Figure 5. Both possibilities do not appear to be feasible in the bulk of the crystal (this is also impossible shortly under the first surface layer) for reasons of lack of space. As it is so much easier, if reaction occurs upon light absorption with the ideally placed neighbour (Fig. 5), the author remindd on several occasions (most recently in [3]) of the fact, that 9,9'-disubstituted head-to-head dimers of anthracenes are thermally labile at room temperature. With this in mind, it appears most likely that indeed the crystallographically expected head-to-head dimer (isomer of 2d) is formed which shortly after decomposes back to 1d. The lattice disturbances thus created may help to regroup molecules in a way that afterwards head-to-tail dimerizations to yield the stable 2d may occur. At any rate, we see long-range transports and the phase rebuilding does govern the reaction of 1d to give 2d. No principal differences to the further examples are discernable. From a molecular point of view it should be stressed that again the molecules stand steeply (67°) in layers which are parallel to (010) (Fig. 5). From these it is easily comprehended that the nanostructures form most easily at crystal faces which stand on (010) where the molecules may escape sideways without being retained. The floes which are built up are of high quality and height (ca. 100 nm). The height appears to relate to the initial step heights on that face.
Thus, for the first time a detailed molecular understanding is obtained through AFM-measurements after a period of decades during which the 1d/2d-problem stagnated and bound considerable research capacities. As AFM allows to follow phase rebuildings far into not anticipated details, these will have to be taken into account from now on. Thus, new fundamentals and new ways of thinking have been created by means of AFM. These offer completely new perspectives for comprehension.
The AFM-technique is in a process of rapid development and enables really new experimental techniques in chemistry, biology, materials research, tribology, and rheology as well as in the production of nanostructures (this at increased force). For progressive crystal studies there is a need for instrumental additions from the side of X-ray diffractometry. For example it would be desirable to have submicro Laue facilities using highly focussed synchrotron X-rays in order to investigate the submicroscopic nanostructures (such as those which are seen in the images of the irradiated surfaces) with respect to orientation and crystal type. The development of such techniques will certainly be fast after further successful AFM studies of chemical reactions in crystals. From the side of AFM stand alone type of instruments, despite their lower resolution capabilities, will allow measurement of very thin and inclined faces, because in this case the crystals need not be mounted directly on the scanner of the AFM (see Fig. 1 in part 1 [1]). This will allow more comprehensive studies of the molecular transport mechanisms on all natural crystallographic faces and on cleavage planes. Molecular interpretations will open up much clearer insights into the dynamics of solid state reactions. Thanks to AFM it is clear that the crystal structure plays a considerably more intimate role for solid-state reactivity than has been hitherto supposed on the basis of the topochemical postulate.
We thank L.O.T. Darmstadt for their contribution to the colour printing costs and the provision of their video printer facilities for the preparation of the colour print patterns. For the determination of the Miller indices we are indebted to Prof. W. Rammensee and Dr. J. Schreuer, Universitaet zu Koeln.
[1] KAUPP, G.: GIT Fachz. Lab. 37, 284 - 294 (1993)
[2] KAUPP, G., TEUFEL, E.: Chem. Ber. 113, 3669 - 3674 (1980)
[4] SCHMIDT, G. M. J.: Pure Appl. Chem. 27, 647 - 678 (1971)
[5] MASON, R.: Acta Cryst. 17, 547 - 555 (1964)
[6] RABAUD, H., CLASTRE, J.: Acta Cryst. 12, 911 - 915 (1959)