LB-films form as monolayers of long chain molecules with a hydrophobic and a hydrophilic end; they are spread at the water/air interface and are transferred to solid supports.
Miller Indices define the groups of parallel lattice planes
with three (at hexagonal lattices with four) bracketed numbers
(hkl) that indicate the reciprocal intercepts of the axes. The
actual crystal faces are to be described morphologically; they
usually correspond to low indexed (hkl) lattice planes. As the
shape of a crystal does in most cases not correspond to the form
of the unit cell (lattice parameters a, b, c, alpha, ß,) and as the parameters may depend on the chosen space group (at
different possibilities for the setting up of the crystal), the
Miller indices of the natural or the cleavage faces of the single
crystal have to be determined via X-ray diffraction and stated
together with the space group. For instance, typical crystals
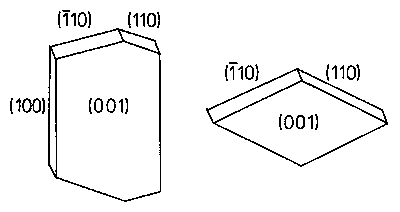
of alpha-trans-stilbene were indexed in space
group P21/a as is shown in the sketch. However, if the space group
is chosen as P21/c, a and c are interchanged and the large faces
of alpha-trans-stilbene are no longer (001) but
(100) under that circumstances.

nm (nanometers) are the millionths of the mm; in the atomic/molecular region Å units are more comfortable: 1 nm = 10 Å.
Phase rebuilding occurs in the initial stage of the solid-state reaction, as long as only few product molecules have to arrange with the basic lattice. As product molecules do not fit geometrically to the lattice they have to wind out and move cooperatively on easy ways, for example along cleavage planes etc. The results are anisotropic surface features that form according to the packing. These features are submicroscopically traced with the AFM.
Phase transformation (transition) occurs after the phase rebuilding, if the concentration in product molecules has increased such that the (rebuilt) basic lattice cannot be maintained. Usually that process leads to a sudden change and forms very large structures in opposition to the preceeding continuous change. The product lattice is formed, still with some distortion by non-transformed molecules. The local product crystals and residual educt crystal detach from each other or the previous crystal disintegrates and fresh surface is created.
Piezos are crystals, ceramics, or polymers that change their elongation if a voltage U is applied, or they create a voltage U if pressure or traction is imposed.
SNOM: Scanning near-field optical microscopy; in USA also the inverted name NSOM is in use. The near-field is established between the tip and the surface which are approached to distances that are much smaller than half of the wavelength of the light. Commercial SNOMs set the distance below 10 nm.
Stereoscopic representations consist of two separate images, one for the right, the other for the left eye. Both images are merged to a 3D-image if viewed from a suitable distance (it is easier if a hand is hold between the eyes). For initial practice various stereoscopes may be used.
STM: Scanning tunneling microscopy detects the tunneling of electrons through dielectrics (air, vacuum, unpolar layer) between an "atomic" tip and a conductor that are put to different electrical potential.
Topochemistry initially included all influences of structure and morphology of solid educts and products (V. Kohlschütter, 1919). Since 1964 it became restricted to a postulate of G. M. J. Schmidt [3], claiming that organic solid-state reactions would proceed with minimal atomic and molecular movements within reaction cavities (M. D. Cohen, 1975). Further influences of the crystal structure were disputed despite opposing experimental evidence. The movements were thought to be restricted to less than 4.2 - 1.5 = 2.7 Å. Numerous exceptions that existed from the beginning were handled with less convincing auxiliary hypotheses. The fact that crystal reactions proceed frequently stereoselectively according to their molecular arrangement but rarely stereospecifically was not acknowledged. However, AFM showed that the topochemical postulate is restricted to very rare topotactic reactions. Non-topotactic solid-state reactions require maximal mobility at the phase rebuilding stage, irrespective of their alleged status as topochemically "allowed" or "forbidden" reactions. That fact is unambiguously proven by the long-range anisotropic molecular movements that give rise to characteristic surface structures. If such mobility is not available, for instance if the molecules are interlocked from all sides, no reaction does occur despite short distances [4,5].
Topotactic organic reactions are very rare. They occur if the molecular shape changes only so minutely that neither the space group nor the lattice parameters (within 4% limits) are altered. Only such single-crystal-to-single-crystal reactions proceed without formation of surface features, as has been shown by AFM [4,5]. The overwhelming number of organic solid-state reactions does proceed non-topotactically in three stages. The sequence of phase rebuilding, phase transformation, detachment and/or crystal disintegration is the major reason for the completeness of crystal reactions.
Prof. Dr. Gerd Kaupp was born in 1940 in Nagold. He studied chemistry since 1959 and got his Ph.D. from the University of Wuerzburg. He had postdoctoral appointments with the Iowa State University in Ames,Iowa in 1996, the University of Lausanne, Switzerland for two years, and the University of Freiburg im Breisgau, where he habilitated in 1973 for organic chemistry. In 1982 he was appointed as full professor in organic chemistry at the University of Oldenburg.
Address of correspondence:
Prof. Dr. G. Kaupp, FB9 - Organische Chemie I - der Universität Oldenburg, Postfach 2503, 26111 Oldenburg;
Fax: Int.+441-798-3409; E-mail: kaupp@kaupp.chemie.uni-oldenburg.de